Endotoxin definition and standardisation
- 分类:新闻资讯
- 作者:Kevin Williams
- 来源:european pharmaceutical review
- 发布时间:2023-02-27 14:09
- 访问量:
【概要描述】Appropriate standards for impurity tests are an important part of analytical testing. In this paper, Kevin Williams outlines various requirements of standards for endotoxin, as stated by United States Pharmacopeia (USP), and elaborates on the definition of endotoxin as distinct from other cellular constituents.
Endotoxin definition and standardisation
【概要描述】Appropriate standards for impurity tests are an important part of analytical testing. In this paper, Kevin Williams outlines various requirements of standards for endotoxin, as stated by United States Pharmacopeia (USP), and elaborates on the definition of endotoxin as distinct from other cellular constituents.
- 分类:新闻资讯
- 作者:Kevin Williams
- 来源:european pharmaceutical review
- 发布时间:2023-02-27 14:09
- 访问量:
Appropriate standards for impurity tests are an important part of analytical testing. In this paper, Kevin Williams outlines various requirements of standards for endotoxin, as stated by United States Pharmacopeia (USP), and elaborates on the definition of endotoxin as distinct from other cellular constituents.
Endotoxin, or lipopolysaccharide (LPS), is a constituent of the outer leaflet (OL) of the outer membrane (OM) of gram-negative bacteria. It is a unique molecule used as a marker of prokaryotic invasion by metazoan immune systems and occurs as a singular constituent among multiple gram-negative bacteria OM constituents, including phospholipids and surface proteins.
LPS is purified away from other impurities as per USP reference standard quality requirements. A highly purified material is used as a standard endotoxin (RSE/CSE), which has served historically to define endotoxin analytically. In industry, there is some desire to prepare a non-purified or “natural standard” for various purposes and to call these preparations “natural endotoxin”. This paper outlines some requirements of standards, as stated by United States Pharmacopeia (USP),1 and elaborates the definition of endotoxin as distinct from other cellular constituents.
Standardisation
An excerpt from USP 40 General Requirements <11>, states:
“Reference Standards provided by the United States Pharmacopeial Convention (USP Reference Standards, or RS) are highly characterised specimens reflective of specified drugs and foods (drug substances, biologics, excipients, dietary supplements, food ingredients, impurities, degradation products, reagents and performance verification standards). When approved as suitable for use as comparison standards for documentary tests or assays (ie, as a monograph component) in the USP or National Formulary (NF), USP RS also assume official status and legal recognition in the United States.”
The “impurity reference standards” section of USP <11> states: “Impurity Reference Standards may be presented as purified single-component materials or as mixtures of more than one impurity.” For industry to maintain the current “endotoxin” standard (for BET <85>), rather than a “gram-negative cell wall” standard, it must be purified and highly characterised, otherwise it will be a “mixed impurity” standard and should be labelled as such.
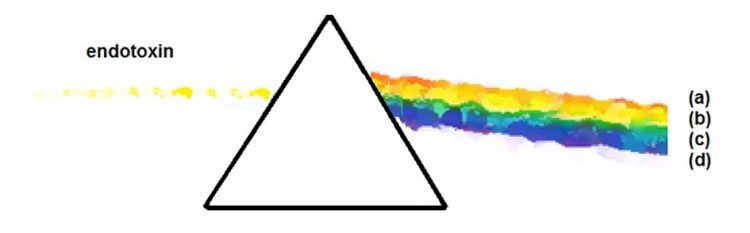
Figure 1: Definition of endotoxin as broken into relevant constituent pieces
Definition of endotoxin
Given the brief nature of this article, only a rudimentary sketch of the definition of LPS can be provided (Figure 1). The topics will be confined to items deemed relevant to endotoxin standardisation:
- LPS biosynthesis
- Reciprocal specificity of the host response
- Asymmetry in the gram-negative bacteria OM
- Contrasting constituents.
1. LPS biosynthesis
Endotoxin is not a random occurrence in nature; it is the product of a bacterial manufacturing process that involves nine separate enzymatic events, followed by export to the gram-negative bacteria cell surface. Unique sugars in LPS include the core sugar, KDO (3 deoxy-α-D-manno-octulosonic acid), and unique arrangements of sugars in the O-antigen moiety, which are used to distinguish bacterial strains when characterising various gram-negative bacteria foodborne illness outbreaks (serotyping).
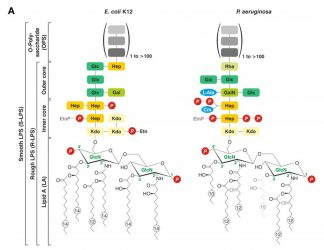
Figure 2: LPS unique structural definition3
The unique LPS structure and associated sugar residues and arrangement are outlined in Figure 2. The enzymatic cascade used to “build” LPS, as encoded in the bacterial genome, is not shown but is extensively detailed by Wang and Quinn and supports that LPS is a specific functional unit.2
2. Reciprocal specificity of the host response
Metazoan systems have already defined endotoxin through their very specific responses. However, not all metazoan responses are the same. For decades, Limulus-based tests have been an effective analytical surrogate for supporting drug testing. The specificity of the mammalian response is extreme. A single molecule of LPS is selected (purified away) from an aggregate by lipopolysaccharide binding protein (LBP)or potentially as a monomer where serum albumin has been found to serve in lieu of LBP.4 Once isolated, the LPS molecule is transferred to MD-2, which sits in TLR4, and brings about a transmembrane signalling event. This is about as “purified” as a molecule can get. TLR4 not only takes the measure of the molecule but also signals a response based upon the underlying variance in sub-molecular moieties.
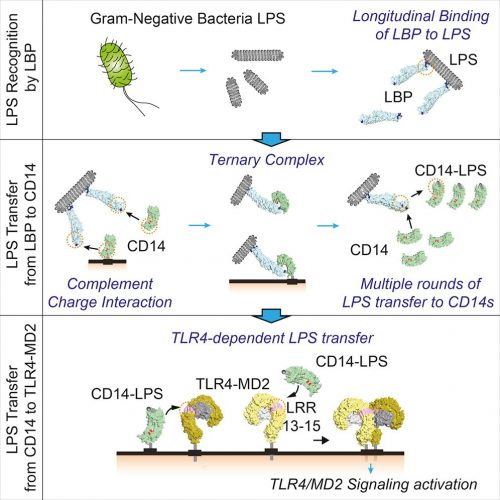
Figure 3: LPS of gram-negative bacteria activates host innate immune responses. Ryu et al. investigated dynamic intermediates in the LBP-CD14-mediated LPS transfer to TLR4-MD-2 down to single-molecule resolution5
To assert that LPS is “not purified” in nature seems a matter of semantics; it begins as a singular molecule in its biosynthesis and ends as a singular molecule in the mammalian receptor. For mammalian detection purposes, it is as purified as it could be by current analytical methods. See an overview of the (canonical) mammalian detection cascade in Figure 3.6
3. Asymmetry in the gram negative bacteria OM
Gram-negative bacteria work, energetically speaking, to maintain the asymmetry associated with the OM. This maintains an efficient barrier against the formation of hydrophobic “patches” that might allow indiscriminate passage of unwanted substances (ie, antibiotics). This is one reason for the difficulty associated with treating gram-negative bacteria infections. If we look at a common textbook diagram of the gram-negative bacteria surface, it is clear where the “asymmetry” occurs (Figure 4).6
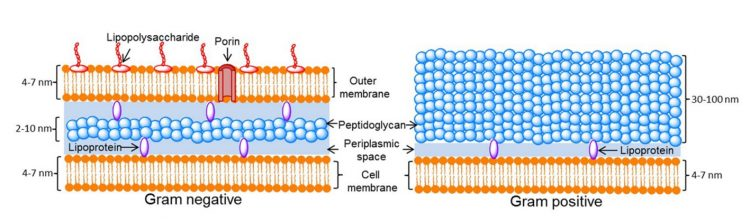
Figure 4: Cell wall structure for gram-negative and positive bacteria6
The asymmetry occurs as the OM OL consists of LPS and the inner leaflet consists of phospholipids (PL).7 If sufficient PLs aggregate in the OL, the cell has specialised methods to reestablish asymmetry; including “floating” in new LPS molecules.8 The longstanding definition of endotoxin as a singular molecular entity includes its unique ability to form this asymmetrical barrier. Thus, the proportionality of PLs and LPS as separate OM constituents (Figure 3) is an important property.
4. Contrasting constituents
It is sometimes easier to define something by describing what it is not, rather than what it is. Endotoxin does not include other membrane-associated molecules. It is a quality or analytical marker for half a dozen bacterial residues that cannot otherwise be detected, and thus a critical quality attribute in pharma manufacturing. One OM constituent type that shares the gram-negative surface with LPS is a “porin”. Porins are protein structures, typically present as trimers, that regulate the passage of water, solutes and nutrients in and out of the cell.
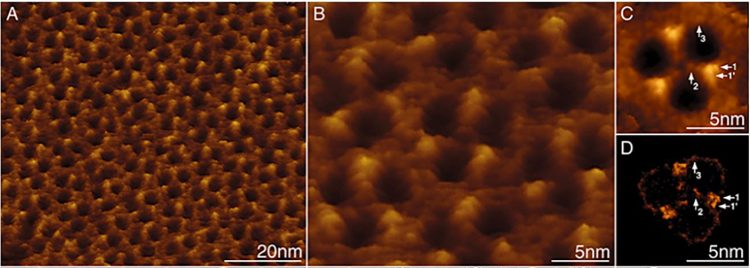
Figure 5: High-resolution analysis of the periplasmic surface of the R. denitrificans OM.
A) Membrane region densely packed with porin trimers.
B) At high-resolution substructure is visible on individual trimers.9
The concept of the gram-negative bacteria OM has evolved recently, as atomic force microscopy (AFM) studies of living bacteria identify holes dominating the gram-negative bacteria surface (Figure 5).9 These woven protein strands form hollow “tubes” that extend from the surface down through the OM and into the cytoplasm (Figure 6D and E). LPS alerts manufacturers to the presence of gram-negative bacteria artifacts via Limulus-based tests; however, porins themselves cannot currently be detected.
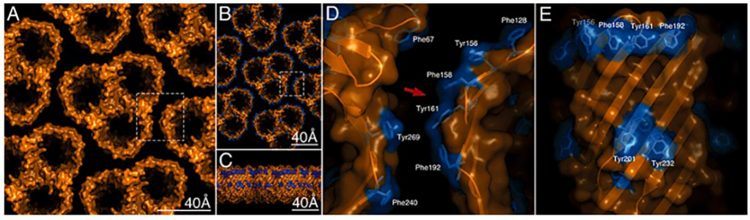
Figure 6: Atomic model of the native supramolecular assembly of porins in the OM9
Using atomic force microscopy (AFM), Oestreicher et al.10 studied two prototypical gram-negative bacteria structures from E. coli and Rhodobacter sphaeroides, stating: “Our research clearly demonstrated that both of these types of cells have an outer surface that is covered in a network of nanometer-sized holes similar to M. magneticum.” Like LPS, porins are separate, enduring structures that are immune reactive and difficult to destroy. “Porins are extremely sturdy proteins that can resist denaturation in the presence of 5M guanidium hydrochloride or two percent SDS at 70°C.”11 Porins should be of interest given that they are immunogenic in nature.12,13
While it doesn’t change the role of LPS as a potent contaminant, it makes it clear that LPS does not represent the entire gram-negative surface, but rather the OM consists of several discrete substances.
Summary
The historical and continued use of purified LPS as a standardised impurity (RSE/CSE) is supported by a detailed definition of endotoxin:
(a) endotoxin is a unique structure with a specialised assembly (nine enzymatic steps)
(b) the specificity of the metazoan response to LPS (via TLR4) is separate from other responses (such as to porins that activate TLR2 or flagella that activates TLR5)
(c) it includes endotoxin’s role in creating and maintaining the asymmetry of the OM in proportion to PLs
(d) its existence as a singular structure separate from and adjacent to other OM structures provides a contrast in terms of structure, function and immune reaction (TLR activation).
Efforts should be made to develop new tests to detect significant non-LPS membrane constituents. This forms a critical distinction. Rather than devising a “natural endotoxin” comprised of unpurified “cell wall” constituents of which only endotoxin can be detected, the individual constituents (endotoxin, phospholipids, porins, flagellin, etc) should have their own assays and standards as a means of broadening microbiological contamination control capabilities. This would provide necessary redundancy, whereas today the entire weight of detecting subcellular gram-negative bacterial artifacts falls on endotoxin.
Appropriate standards for impurity tests are an important part of analytical testing. As per USP <11>, an impurity mixture labelled as a “natural endotoxin” is not endotoxin, but a mixture that may contain endotoxins, proteins, phospholipids, nucleic acids and porins. Today’s Limulus-based tests are specific for endotoxin, not for cell wall or cytoplasmic constituents that are not endotoxin; this specificity is inherent in both Limulus Factor C and mammalian TLR4 detection architecture. The definition of endotoxin is a cross-discipline definition and not one that can be casually re-defined.
References
1. USP40-NF35 (Official as of 1-May-2018)
2. Wang X, Quinn, PJ. Lipopolysaccharide: Biosynthetic pathway and structure modification. Progress in Lipid Research 2010; 49: 97–107.
3. Ranf S. Immune Sensing of Lipopolysaccharide in Plants and Animals: Same but Different, PLOS Pathogens 2016.
4. Esparza et al. Endotoxin-albumin complexes transfer endotoxin monomers to MD-2 resulting in activation of TLR4, Innate Immunity, Innate Immun. 2012. 18(3): 478–491.
5. Ryu et al. Reconstruction of LPS transfer cascade reveals structural determinants within LBP, CD14, and TLR4-MD2 for efficient LPS recognition and transfer. Immunity 2017. 46, 38–50.
6. Berezin et al. Replacing a Century Old Technique – Modern Spectroscopy Can Supplant Gram Staining. Scientific Reports 2017. 7: 3810.
7. Henderson et al. The Power of Asymmetry: Architecture and Assembly of the Gram-Negative Outer Membrane Lipid Bilayer. Annu. Rev. Microbiol. 2016. 70:255–78.
8. Ursell et al. Analysis of Surface Protein Expression Reveals the Growth Pattern of the Gram-Negative Outer Membrane. PLoS Comput Biol., v.8(9); 2012 Sept. PMC3459847`
9. Jaroslawski et al. High-resolution architecture of the outer membrane of Gram-negative bacteria Roseobacter denitrificans, Molecular Microbiology 2009. 74(5), 1211–1222.
10. Oestreicher, Taoka, and Fukumori. A comparison of the surface nanostructure from two different types of gram-negative cells: Escherichia coli and Rhodobacter sphaeroides, Micron 2015. 72, 8–14.
11. Koebnik R, Locher KP, Van Gelder P. Structure and function of bacterial outer membrane proteins: barrels in a nutshell, Mol Microbiol. 2000 Jul;37(2):239-53.
12. Liu et al. Immunogenic characterization of outer membrane porins OmpC and OmpF of porcine extraintestinal pathogenic Escherichia coli. FEMS Microbiol Lett 2012. 337: 104–111.
13. Pérez-Toledo M. Salmonella Typhi Porins OmpC and OmpF Are Potent Adjuvants for T-Dependent and T-Independent Antigens. Front Immunol. 2017. 8: 230.
扫二维码用手机看
产品推荐





底部联系方式信息(左)
400-687-1881
公司电话:010-87875910
公司邮箱:info@bio-life.cn
公司官网:www.acciusa.cn www.bio-life.cn
公司地址:北京市大兴区中关村科技园区大兴生物
医药产业基地华佗路50号院18幢(中国药谷CBP)

关注官方微信

关注手机网站





